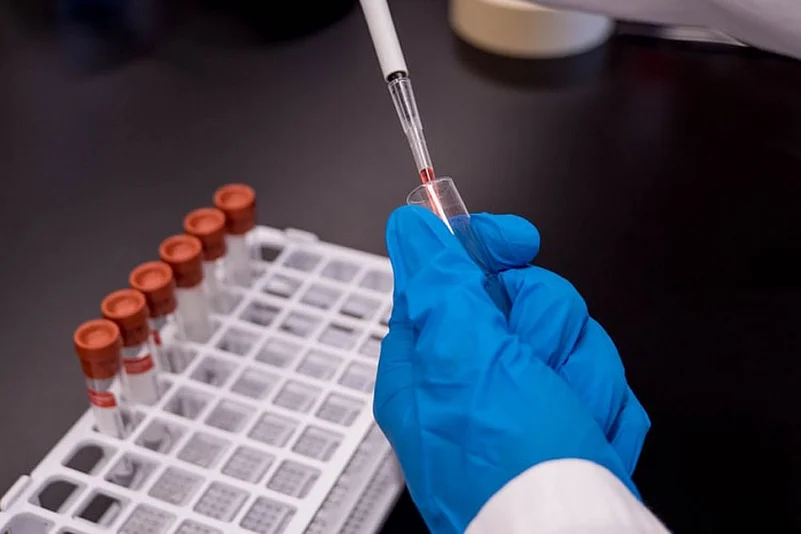
The early-stage human trial data of a COVID-19 vaccine developed by AstraZeneca and Oxford University reveals that it is safe and induces immune response, with mild side effects in some participants, which the scientists say can be treated with the commonly available pain medication paracetamol.
The preliminary results of the phase I/II trial, published in The Lancet journal, involved 1,107 healthy adults, and found that the vaccine induced an immune response both via antibodies and the T cells of the immune system up to day 56 of the ongoing trial.
"The Phase I/II data for our coronavirus vaccine shows that the vaccine did not lead to any unexpected reactions and had a similar safety profile to previous vaccines of this type," said Andrew Pollard, Chief investigator of the Oxford Vaccine Trial at Oxford University.
According to the researchers, the immune responses could be even greater after a second dose of the vaccine, as found in a sub-group of 10 participants.
Commenting on the study, Eric Feigl-Ding an epidemiologist at Harvard University, who was unrelated to the research, said the vaccine did not produce any adverse effects.
In the study, the scientists said compared to the control group, the SARS-CoV-2 vaccine caused minor side effects more frequently, however added that some of these could be reduced by taking paracetamol.
"No serious adverse effects. Acetaminophen, aka paracetamol, reduced any mild effects. Neutralising antibodies induced in all patients after second dose. Neutralising antibody responses correlated strongly with antibody levels," Feigl-Ding noted in a Tweet.
They said there were no serious adverse effects from taking the vaccine.
Fatigue, and headache were the most commonly reported reactions from the participants given the vaccine, the study noted.
It said the vaccine produced detectable T Cell response within 14 days of vaccination, and an antibody reaction within 28 days.
The researchers said the antibodies could neutralise the virus and prevent it from infecting cells, adding that these are important for protection.
According to the scientists, the antibody responses were the strongest in the patients after they received a booster dose.
They said with the booster dose, 100 per cent of the participants' blood had neutralising antibodies which could block the virus.
"We saw the strongest immune response in the 10 participants who received two doses of the vaccine, indicating that this might be a good strategy for vaccination," Pollard said.
According to the researchers, the trial began in April testing the Oxford coronavirus vaccine ChAdOx1 nCoV-19.
They said the scientists started working to develop a vaccine against the global threat that is coronavirus in January 2020, and have been working with unprecedented urgency in a race against the coronavirus.
During the phase I/II trials, the researchers said the vaccine has been evaluated in more than 1,000 healthy adult volunteers aged between 18 and 55 years in a randomised controlled trial.
According to a statement by the scientists, a subset of these volunteers -- 10 people -- received two doses of the vaccine.
They said between April 23 and May 21, 1077 volunteers, received the vaccine ChAdOx1 nCoV-19 or a placebo MenACWY vaccine, which is a meningitis vaccine.
"There were no serious adverse health events related to ChAdOx1 nCoV-19," they noted.
While the study proved there was an immune response produced via the vaccine, the scientists said the next step is to prove that it protected against SARS-CoV-2.
"The immune responses observed following vaccination are in line with what previous animal studies have shown are associated with protection against the SARS-CoV-2 virus, although we must continue with our rigorous clinical trial programme to confirm this in humans," Pollard said.