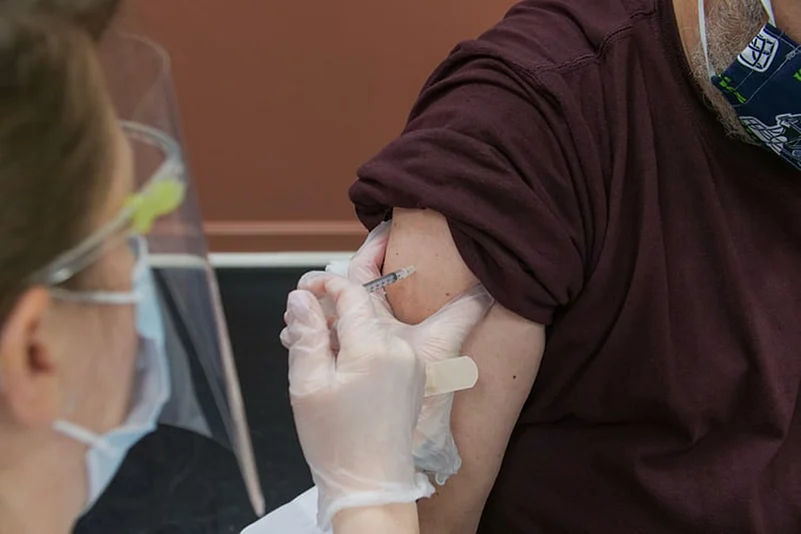
Amid the European Union approving the Moderna Covid-19 vaccine for children aged 12-17, AIIMS chief Dr Randeep Guleria on Saturday said that India is mulling to begin vaccinating children by September.
According to a report published by NDTV, Dr Guleria stated that such a move will prove crucial in breaking the chain of transmission of Covid-19 and curbing the spread of the virus in the country.
“I think Zydus has already done the trials and they're waiting for the emergency authorisation. Bharat Biotech's Covaxin trials should be over by August or September, and by that time we should get an approval. Pfizer vaccine has been already approved by the FDA (US regulator - Food and Drug Administration). Hopefully, by September, we should start vaccinating children, and that would be a big boost as far as breaking the chain of transmission is concerned," NDTV quoted Dr Guleria as saying.
This development comes in the backdrop of the European Medicines Agency authorising Moderna's Covid-19 vaccine for children aged 12 to 17, the first time the shot has been authorized for people under 18.
In a decision on Friday, the EU drug regulator said research in more than 3,700 children aged 12 to 17 showed that the Moderna vaccine — already given the OK for adults across Europe — produced a comparable antibody response.