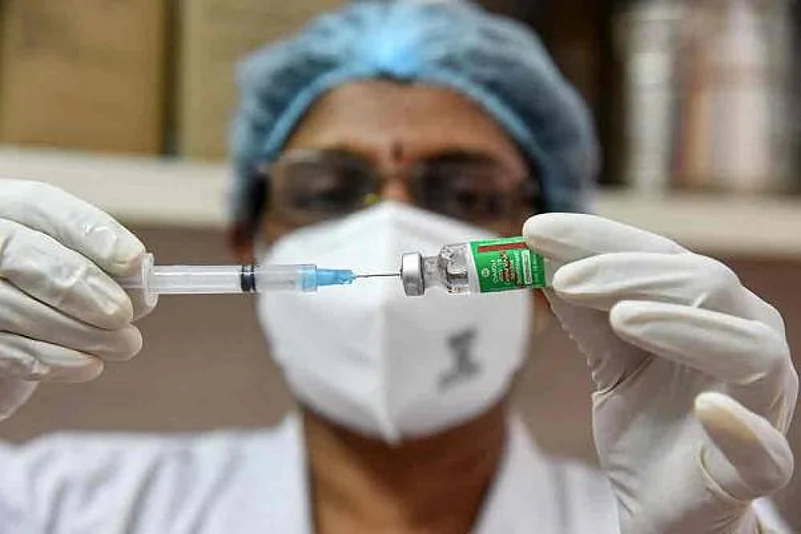
Pakistan has approved the emergency use of the Oxford-AstraZeneca Covid-19 vaccine. As the coronavirus tally surged to 519,291 in Pakistan, the government hopes to make the drug available by the first quarter of the year.
According to a report in the Geo TV, Dr Faisal Sultan who is Special Assistant to Prime Minister Imran Khan on Health confirmed on Saturday that the Drug Regulatory Authority Pakistan (DRAP) has granted approval for emergency use of the Oxford-AstraZeneca coronavirus vaccine across the country.
Minister for Planning Asad Umar, who is also the chief of National Command and Operations Centre (NCOC), the country's coronavirus control body, said that the vaccine will be rolled out by March, the report said.
In the first phase, the vaccine will be given to health workers and those aged 65 years and above, Umar said.
The vaccine is designed by scientists at the University of Oxford and produced by AstraZeneca, a British-Swedish multinational pharmaceutical and biopharmaceutical company.
Pakistan reported 43 deaths in the last 24 hours, taking the total number of fatalities due to the disease to 10,951, according to the Ministry of National Health Services.
The total number of Covid-19 cases in the country reached 519,291 after 2,521 new infections were detected in the past 24 hours.
Dr Sultan said on Friday that Pakistan was in touch with China and other companies to purchase the Covid-19 vaccine.
He said trials of the vaccine produced by the Chinese firm, Cansino, were near completion and if its tests were found to be successful, the government would register the medicine with the DRAP for procurement.
He said international coalition Covax had announced that it would provide Pakistan 50 million free doses of Covid-19 vaccine.
“As 100pc population cannot be vaccinated in any country, we need to target 70pc of the vaccineable population, which is 70 million,” he said.
With PTI inputs