There is no vaccine against the venal mind. No immunisation ever invented gives us a complete coat of armour. The law is only good enough to catch the more obvious type of visible corruption. When it’s raised to a more abstract and institutionalised level, where it forms the very operating logic of a system that surrounds you with good words, it simply becomes the natural order of things. But once in a while, a crack develops in the consensus and the light filters through. The evening of January 20, when the lonely dissenting voice of Dr Vipin Vashishtha was sought to be banished by the Indian Academy of Paediatrics (IAP), was one such moment. What showed through in that light was the entire unholy architecture of India’s immunisation programme.
The evening did not go well for Dr Vashishtha, ex-convenor of the academy. In the late hours, his fellow members had him thrown out unceremoniously from the IAP general body meeting. The reason: Dr Vashishtha had blown the whistle on the silent collusion of interests between paediatricians and vaccine manufacturing companies. It’s a nexus that enables these companies—Indian and multinational—to push expensive vaccines into the market, some of them not even answering to a real need. The market is worth thousands of crores, and booming. And doctors make unwarranted profits in the process, at the cost of the unknowing public.
The IAP, the nodal private sector body, directly influences 10-15 per cent of all immunisation in India—and though that makes it seem limited in scope, this is only in terms of volumes. In value terms, the market is almost as big as the state-run immunisation programme. And the IAP’s charter of immunisation, followed by all private paediatricians, exists like a quasi-official model of healthcare to be aspired to by everyone. Immunisation via the public health agencies is a more regulated territory, but still the creeping influence on it is not hard to divine—this is because the overarching coalition of global interests that pulls the strings from remote boardrooms is the same on the public as well as private sector.
Healthcare is a transaction of trust. Immunisation is one of the first steps in that transaction in an individual’s life. But the field has become so grey that vaccines are being sold for diseases not even prevalent in India! Take yellow fever vaccines. A well-known vaccine distributor in Delhi says some 2,000 units of yellow fever vaccine are sold every month in India. Yellow fever has zero incidence in India (or Asia)—while being prevalent in Africa and Latin America—and the vaccine is only needed to be administered to individuals who travel overseas. Each dose of the vaccine costs Rs 1,850 to the patient.

For Dr Vashishtha, it’s been a long battle against this tacit compact; the January 20 general body meeting was the last straw. It all began in 2011, when he took over as the IAP convenor. The IAP, a national association of paediatricians, is responsible for making recommendations for new vaccines that enter the market: this becomes the benchmark adhered to by paediatricians across India. The government too consults it to update its own national immunisation programme.
The revelations made by Dr Vashishtha have now reached Parliament. On March 17, Dausa MP Harish Meena raised a starred question in the Lok Sabha on the subject. In his reply, Union health minister J.P. Nadda admitted the government was aware of the corruption within the IAP and had also received a complaint from a doctor in Karnataka on it. The government “does not endorse the recommendations” of the IAP, Nadda added, and “all vaccines included in the Universal Immunisation Programme (UIP) are available free of cost across government health facilities.” Yet, no sign there of any move to address the lack of regulations or of any inclination to introduce a structure of guidelines.
India vaccinates over 27 million newborns every year—a 10 per cent ratio means 2.7 million of them get vaccinated through the private sector. Naturally, in urban areas, the ratio is much higher. “Over 40 per cent of children immunised in cities are taken to private hospitals,” says Pradeep Haldar, chairman, National Technical Advisory Group on Immunisation (NTAGI), the government body responsible for making suggestions for vaccines to be introduced into the country.
The essential issue is the structure of the industry. Most vaccines are sourced from foreign players with limitless resources. And they are willing to spread it around a bit among doctors and distributors with a single agenda: to increase sales. Not only does this bring about huge price mark-ups, it also pushes ‘cash vaccines’—those that make big bucks but may not be the safest or the most needed for disease prevention. And when reputed bodies of paediatricians and other groups offer their stamp of approval, they are basically acting as lobbyists—though in the guise of arbiters acting in the name of public good.
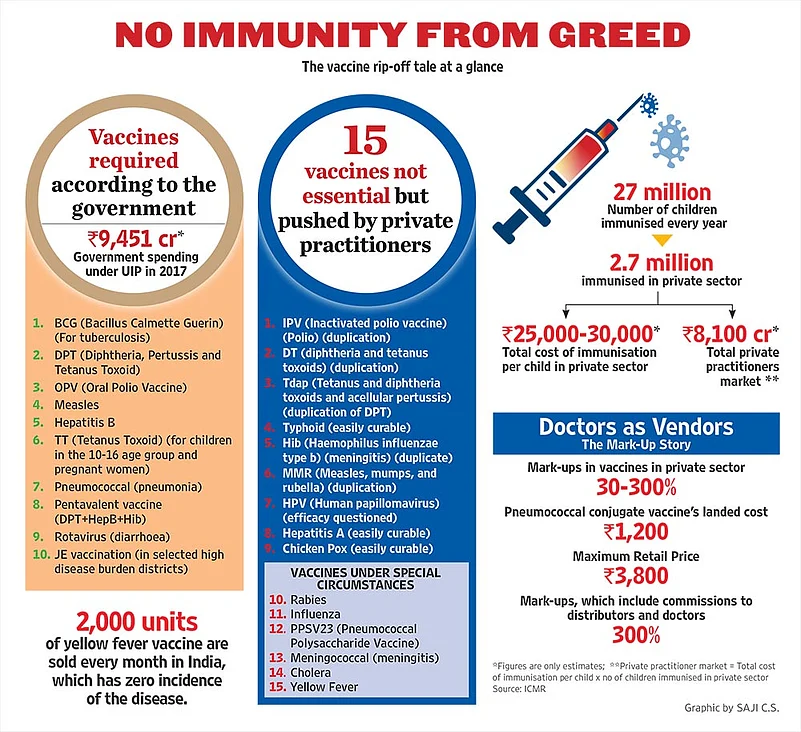
Consider this. The UIP, which is the government programme targeted at immunising all children up to age five against certain diseases, offers a range of six vaccines across India. In addition, there are three more state-specific vaccines. A private doctor, on the other hand, may offer you a range of 25 vaccines for your child! The costs involved may seem tolerable for a lot of city parents eager to ensure a healthy baby; the volumes bring in the profits, which are substantial. Dr Bakul Parekh, paediatrician and IAP secretary general, says a parent can spend anywhere up to Rs 25,000-30,000 in the full course of vaccination for their newborn. Count also the consultation fee charged by doctors for every dose, for almost one visit to the doctor every month till the child turns 12. Now compare this to the UIP, which is offered to all children free of cost.
Under the Drugs and Cosmetics Act, 1945, paediatricians are required to maintain records of every vaccine dispensed by them, to ensure only registered medical officers dispense or prescribe such vaccinations. Outlook asked several paediatricians for such records but they were unwilling to provide them. Moreover, there is no government or non-governmental authority where such records need to be submitted by paediatricians. This unregulated scenario has opened a huge market for non-authorised doctors to sell such vaccines.
The biggest motivating factor here is the undue mark-ups offered to every intermediary. Studies suggest mark-ups can range from 30-300 per cent. Outlook obtained several documents from distributors and doctors laying bare the scale involved. Take the pneumococcal conjugate vaccine, administered to prevent pneumonia. It costs the parent Rs 3,800 per dose. And the landed cost of the vaccine, meaning the amount it’s imported for, is only Rs 1,200 per dose. In other words, between distributor and doctor, that’s a neat mark-up of over 300 per cent.
Compared to pharmaceuticals and medical devices—which are need-based, curative aids—the vaccine market operates on a different logic, because these are by definition preventive interventions and irrational fear can be a factor. Often, the very existence and supply of a vaccine can engineer demand. “Several new vaccines are introduced in India that may or may not be required by all children. There is no objective way to prioritise new vaccine introductions. Ideally, it should be guided by our local burden of disease and needs. But companies push these vaccines just to earn higher profits and many paediatricians collaborate with them,” says Yogesh Jain, founder of Jan Swasthya Sahayog and a member of the National Health Mission steering group.
The mere awareness of a disease, whether prevalent in India or not, becomes a coercive tool for doctors to prescribe costly vaccines that might not be needed. The UIP takes into account geographical variations—the prevalence of Japanese encephalitis, for instance, is limited to certain states—and most vaccines it prescribes are essential for the well-being of a child. The private sector, though, pushes through a whole bouquet of vaccines without any empirical data to back their need or effectiveness. Dr Jacob Puliyel of St Stephens Hospital, New Delhi, and a member of the NTAGI board, elucidates this with the example of the pneumococcal vaccine. “The vaccine used to eliminate pneumonia targets only 10-13 strains of the disease, which is known to have more than 100 strains,” he says. “If you do the math, it means the vaccine is capable of preventing pneumonia only in four out of hundred children,” he adds.
So how do such vaccines enter the Indian market to begin with? According to paediatricians, this happens through a well-oiled system of quid pro quo between vaccine companies and organisations responsible for recommending vaccines. “Most doctors depend on the recommendations given by organisations such as the IAP,” says Dr Vashishtha. And many IAP members, who chair discussions on what vaccine to recommend, have associations with companies who appease them through gifts and several other soft bribes, he adds.
Several doctors told Outlook on the condition of anonymity that once a certain vaccine is cleared by the Drug Controller General of India (DCGI) for use in the country, representatives from different multinational vaccine companies visit doctors to push their products. This is done by offering not only extremely lucrative mark-ups on vaccines but also other incentives, like covering travel expenses to exotic locations for medical conferences and free samples of vaccines the company would like to promote. The IAP itself is almost completely funded by private players, a look at their website confirms. The organisation receives hefty donations to the tune of crores from several companies, both Indian and foreign. Online records show that, in 2016, IAP earned Rs 5.5 crore and yet did not show any profits in its financial documents.
A 2012 paper published in the Indian Journal of Medical ethics—titled ‘Financial incentives and the prescription of newer vaccines by doctors in India’, by Dr Rakesh Lodha and Anurag Bhargava—also records the mark-ups in newer vaccines. The common Haemophilus influenzae type b (Hib) conjugates vaccine, for instance, has an MRP of Rs 426 and is supplied to doctors at Rs 251, a discount of 69 per cent per dose for the doctor. The information for the paper was obtained through communication sent to a doctor by distributors. “The significant financial incentive being offered to doctors on dispensing newer and combination vaccines alters the nature of the relationship between doctor and patient and opens a wide area of conflict of interest: the doctor benefits significantly by prescribing a particular vaccine whereas the benefit to the recipient may be marginal,” concludes the paper.
When Outlook got in touch with Dr Bhargava, a professor at the Yenepoya Medical College in Mangalore, he said such mark-ups are commonplace within the industry. He attributes it to the shutting down of public sector vaccine manufacturers in 2008. “The shutting down of PSUs manufacturing vaccines has allowed private companies to take advantage of a market without regulation or a cap on prices, which enables them to further push vaccines through high mark-ups,” he says.
The cold chain used in transport poses another issue. Most vaccines need to be stored under controlled temperatures of 2-8 degrees Celsius; exposure to temperatures above that can spoil it. In the private sector, the cold chain is maintained by MNC suppliers themselves and the quality standards can be dubious. Walk into Bhagirath Palace in Chandni Chowk, home to one of the biggest drugs wholesale markets in Delhi, and you see vaccines being transported from trucks to individual shops without adequate refrigeration, breaking the cold chain. Dr Davinder Gill of Hilleman Laboratories, a vaccine developer, says no real data is available on how much may have perished. “Wastage statistics are hard to get in the private sector. No manufacturer makes such records public,” he says.
One way of telling whether a vaccine is spoilt or not is the labels, which change colour if the vaccine inside is spoilt, says Dr Gill. The system may have utility, especially in the cities, but smaller towns still face the threat of spoilt vaccines being administered. Outlook sent a detailed questionnaire to several companies in this regard but got no response. We also got in touch with a distributor who, while requesting anonymity, admits faulty vaccines could still be distributed in smaller towns and villages via non-licensed stockists unaware of proper cold storage methods. “In cities, if a vaccine gets spoilt, it’s usually returned to the company but in smaller towns and villages, such vaccines slip through the systems and may be administered to patients.”
The IAP and individual practitioners have been trying to grapple with the issues at some level. Several doctors who attended the IAP’s annual medical conference in Bangalore last year tell of how company representatives present there distributed gold coins to doctors who bought a certain amount of a new vaccine. To combat this problem of co-dependence creating undue advantage, the IAP has put in place regulations for declaration of conflict of interest. “Each meeting for recommendation of vaccines under the IAP is first reviewed by a committee and then voted upon,” says Dr Parekh.
Several high-level members attend such meetings, bringing in conflict of interest. Vashishtha says he has witnessed this network first hand—while preparing the schedule for the 2016 recommendations, two doctors, Dr Anupam Sachdeva and Dr Ajay Gambhir, did not submit their conflict of interest forms and the effect was visible. Recommendations to include a vaccine of a particular company, voted on unanimously, were taken back by the then IAP president under the influence of Dr Sachdeva, the current IAP president, Vashishtha alleges.
“A vaccine from the company Biomed was put in the schedule after deliberations. Yet, under Dr Sachdeva’s influence, the recommendations carrying this vaccine were withdrawn. To make matters worse, Dr Sachdeva also did not submit his conflict of interest form,” says Dr Vashishtha, adding that Sachdeva wished to promote a similar vaccine manufactured by the company Bharat Biotech instead. An Indian company, Bharat Biotech did not respond to Outlook’s queries despite several attempts. Bharat Biotech was also the principal sponsor of the paediatricians’ conference at Bangalore and has paid over Rs 1.5 crore to the IAP. The same company allegedly distributed gold coins as freebies to doctors in the conference.
When Outlook got in touch with Dr Gambhir and Dr Sachdeva, they rubbished the allegations against them, stating the required declaration of conflict of interest had been duly submitted and yet they were being targeted by Dr Vashishtha. Dr Gambhir in turn alleged the IAP has several members who do take grants and bribes from foreign organisations. “The influence of the foreign lobby is immense in the IAP. Several doctors take grants and bribes from such companies, including all-expenses-paid foreign trips. This corruption has been industrialised as many of these companies use IAP as a front to recommend such vaccines to the public for consumption,” he says. Dr Sachdeva too says the IAP takes several grants as well as funds from vaccine companies for the running of the organisation—a clear signal of a conflict of interest.
The lack of guidelines and credible regulation, either at the organisation’s level or from the government, is clearly the core issue. The immunisation subcommittee of the IAP has issued guidelines for the use of some vaccines—these may not be required by all children and have been placed in the category of “vaccines to be administered after one-to-one discussion with the parents”, as there are insufficient epidemiological grounds for their routine administration. Yet, the guidelines remain vague and open to interpretation and, given the significant financial inducements, one can fairly predict the eventual picture.
The government too has no well-evolved guidelines for vaccines or code of conduct for those administering them. According to existing guidelines, a new vaccine can enter the country simply through a study on 60 people presented to the DGCI, provided it’s licensed in any other country. There is no need for firms to establish disease burden or even efficacy of the vaccine. The health ministry simply offers no set regulations for the administration of vaccines or for controlling unneeded vaccines in the private sector. The strange thing is, no such move towards an ombudsman-like role seems to be on the anvil either. This has left open a huge lacuna in a vital area of health delivery. On one side, the prices follow a kind of laissez faire. And on the other, a huge country like India remains vague and open-ended even about the list of vaccines actually required.